Donate TodayEnroll Now
What’s needed to solve the vitamin D deficiency and type 1 diabetes (T1D) epidemics?
- Measure the 25-hydroxyvitamin D serum levels, Omega-3 Index, AA:EPA ratios, hs-CRP, HbA1c, and T1D autoantibodies levels every 3-6 months along with an online health survey
- Provide education about supplementation, diet, UVB exposure, and other lifestyle changes to get vitamin D levels to at least 40-60 ng/ml (100-150 nmol/L), improve omega-3 status with an AA:EPA ratio of <3, and maintain an HbA1c level of 5.5% or below
Inflammation Panel & T1D Autoantibody Home Blood Spot Test
An increasing body of evidence has shown that chronic inflammation can cause or advance many common chronic diseases, such as diabetes, with higher levels of cellular inflammation indicating a greater risk of the development of and/or accelerated progression towards chronic disease. The following combination of tests can be used to help track and manage inflammation.
 Vitamin D
Vitamin D- Omega-3 Index with AA:EPA and Omega-6:Omega-3 Ratios
- HbA1c
- hs-CRP
As part of this project, participants will also test for the presence of
- Anti-Insulin (also known as mIAA or IAA)
- Anti-IA2 (IA-2A)
- Anti-GAD65 (GAD65 or GADA)
- Anti-ZnT8 (ZnT8)
This project will help identify potential changes in T1D diagnosis and autoantibody status that may result from vitamin D and omega-3 fatty acid testing and education, as well as provide additional education about T1D autoantibodies, inflammation, and other T1D related information. Watch this video for more information on participating in the project and testing with the home blood spot test kits.
Join this project now to measure these T1D autoantibodies along with other essential markers for yourself or your child.
Order Your Test Kit & Enroll Here
Interested in more information?
Complete this short contact form, and our study coordinator will be in touch with you.
 Who is at risk of developing T1D?
Who is at risk of developing T1D?
Family history is an indicator of T1D risk, however, other factors are associated with the development and progression of T1D, such as inflammation, autoantibodies, vitamin D3 deficiency, even viral infection. The presence of islet cell autoantibodies indicates an autoimmune response against the cells of the pancreas, and is strongly associated with development of T1D. Specific T1D autoantibodies are
- Anti-Insulin (mIAA, IAA)
- Anti-IA2 (IA-2A)
- Anti-GAD65 (GAD65, GADA)
- Anti-ZnT8 (ZnT8)
- ICA512 (previously thought to be relevant, no longer considered)
Improved vitamin D3 and long-chain omega-3 status, as well as decreased inflammation, have been associated with lower levels of these autoantibodies and therefore lower risk of T1D development.
Are You Interested in Sponsoring a Participant?
Please consider a donation towards a scholarship fund to help children and their families gain access to this opportunity to receive free testing and help prevent T1D. The fees associated with the testing in this program (3 test kits over a span of approximately 18 months, at a cost of $315 plus shipping per kit) may not be affordable for everyone interested, and we highly encourage donations to help sponsor participation. Can you sponsor a qualifying participant in the study? Even sponsoring a single test kit could make a big difference for someone at risk.
T1D Prevention Project Study Coordinator
 Our study coordinator is likely one of the most knowledgeable individuals on this topic… check out her story below! GrassrootsHealth is excited to be working on this project with Sonia Chritton, as she brings years of experience, research, and involvement with the protocol and within the T1D community.
Our study coordinator is likely one of the most knowledgeable individuals on this topic… check out her story below! GrassrootsHealth is excited to be working on this project with Sonia Chritton, as she brings years of experience, research, and involvement with the protocol and within the T1D community.
Schedule a call with Sonia to discuss the project here.
The Research
An increasing body of evidence has shown that chronic inflammation can cause or advance many common chronic diseases (1), with higher levels of cellular inflammation indicating a greater risk of the development of and/or accelerated progression towards chronic disease. Type 1 Diabetes (T1D) is one of the diseases that are highly affected by inflammation (2,3).
It is known that progression to many forms of diabetes, including T1D, can be predicted by measuring levels of inflammation. Research has also shown that vitamin D and omega-3 fatty acids are both anti-inflammatory (4,5), therefore, progression of T1D could be predicted by measuring levels of inflammation using such blood spot tests as 25(OH)D (6,7,8) and the ratio of AA:EPA (9). 48 international vitamin D researchers have recommended a vitamin D serum level of 40-60 ng/ml (100-150 nmol/L) for the prevention of many chronic diseases, while a AA:EPA ratio of <3 is a target for the most reduced rates of chronic inflammation.
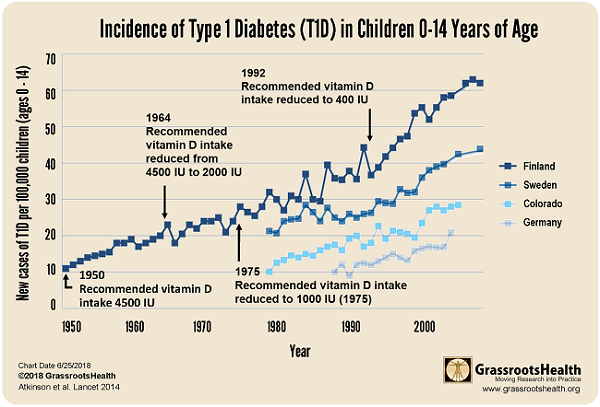
Numerous studies show that intake of vitamin D and omega-3 fatty acids reduces inflammation (10,11). A number of health conditions related to low vitamin D have also been correlated to low levels of omega-3 fatty acids (12-21). Anecdotal evidence suggests a role of vitamin D and omega-3 fatty acids in possibly reversing or stopping this progression. (5,22) Epidemiologic studies show that children given Cod Liver Oil, which contains both vitamin D and omega-3 fatty acids have significantly reduced rates of diagnosis of T1D. (23,24,25)
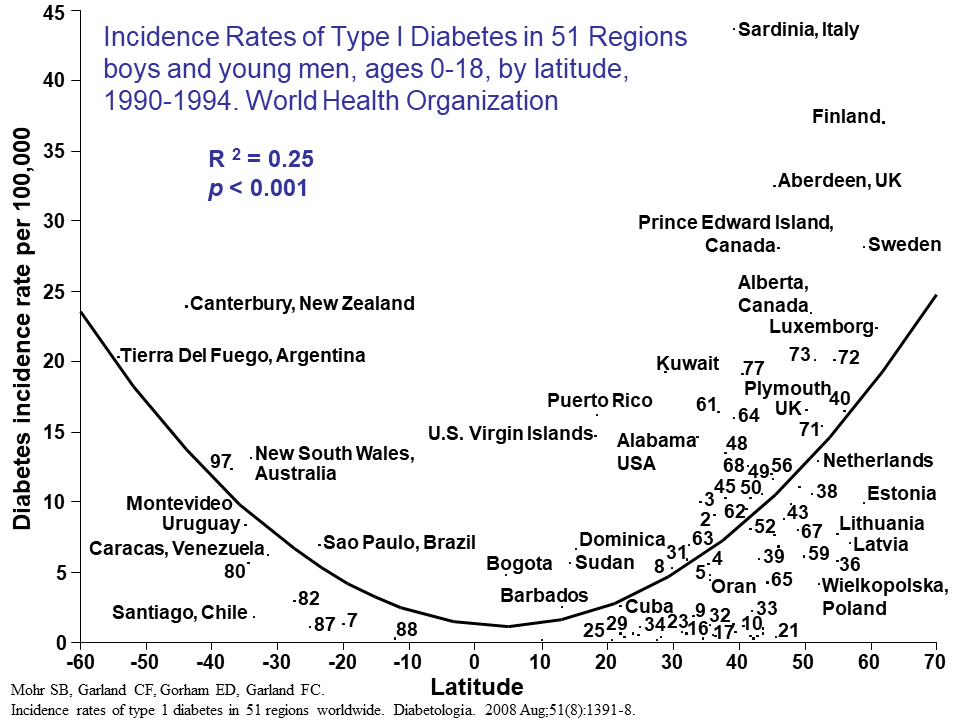
Studies have also examined the incidence of T1D in countries at different latitudes, and found that countries further away from the equator, where there is less UVB available to produce vitamin D in the skin year-round, have a higher incidence of T1D.
Omega-3s are often found in foods that are also naturally high in vitamin D (such as fatty fish). There is very little research available on the combined effect of vitamin D and omega-3 fatty acid intake and resulting blood levels on specific health outcomes. Incorporating omega-3 education and testing (using omega-3 status testing such as the Omega-3 Index and the ratio of AA:EPA (27,28)) along with vitamin D education and testing will help shed light on such a combined effect. It will also allow us to study levels of both nutrients independent of consumption since the effect of intake on blood status will vary from person to person, and it may take different doses to reduce inflammation if a person has a diet high in AA. The ratio of omega-6 (pro-inflammatory) to omega-3 (anti-inflammatory) fatty acids in humans has changed from 1:1 to 20-30:1 over time (4,29,30).
This study sub-set will help identify potential changes in the progression towards a T1D diagnosis that may result from nutrient status testing and education of both vitamin D and omega-3 fatty acids, as well as provide additional education about T1D autoantibodies, inflammation and anti- and pro-inflammatory foods, and other T1D related information.
Check Now!
Register to have your vitamin D and Omega-3 Index with AA:EPA ratio and T1D autoantibody levels tracked every 3-6 months, and be an important part of this project to solve the type 1 diabetes epidemic.
Join this project now to measure these T1D autoantibodies along with other essential markers for yourself or your child.
Order Your Test Kit & Enroll Here
Interested in more information?
Complete this short contact form, and our study coordinator will be in touch with you.
Incidence of Type 1 Diabetes Rises 3-5% Each Year
T1D has been diagnosed in approximately 1.3 million people in the U.S., including about 200,000 young people under 20 years old. The incidence continues to increase 3-5% annually. By 2050, the expected prevalence of T1D is estimated to be 5 million people in the U.S., including 600,000 young people. Expected lifetime medical expenses and income loss due to diabetes total approximately $643 billion in the U.S. (2016).
Download to Print & Share
Vitamin D and Type 1 Diabetes – Frequently Asked Questions
D*action WIRB Approved Study Protocol
Project Brought to You by:
23+ Year Delay: A T1D Prevention Protocol Success Story
Sonia Chritton, PreventT1D.org
I believe the most cathartic way of dealing with a life changing event is to dig in and get involved!
Shortly after my son was diagnosed with T1D, we enrolled his older brother and his younger sister in two observational studies. The goal was to learn his genetic risk by finding out his allele combination. The highest risk alleles are DR3 and DR4. You get one from each parent – so nobody can inherit T1D from “his or her” side of the family. I also wanted to learn whether or not they had started developing autoantibodies. The autoantibodies are IAA, GADA, IA-2A and ZNT8.
While on a volunteer assignment reviewing grants, I read a powerful proposal articulating the correlation between inflammation and T1D. The timing was critical because shortly after that, I received a call from the head of the study my eldest son was enrolled in letting us know that he was positive for two autoantibodies. I immediately thought of that amazing grant and compelling logic. We found that Ben not only had the autoantibodies confirmed, but his level of inflammation was high.
I was desperate to find out what we could do to stop progression. One child with T1D was enough!
In the grant proposal, they were suggesting fish oil. This would be the key component in our attempt to delay progression. As a mom, I figured it couldn’t hurt to reach out to brilliant medical professionals in the field of T1D prevention. I asked:
“If this was your child, what would you try?”
Here was their feedback that led to “Ben’s cocktail”
- Docosahexaenoic Acid (DHA) in a relatively high dose (40 mg/kg to 120 mg/kg of body weight). DHA works to suppress inflammation. You can supplement with DHA rich foods (wild caught salmon, etc.), but this alone won’t give the dosage likely needed if already autoantibody positive. You can get an algal version that is DHA only, but fish oil which also contains EPA works too. Just focus on the DHA and EPA numbers on the label of whatever source you are using.
- http://www.ncbi.nlm.nih.gov/pubmed/25039804
- http://www.ncbi.nlm.nih.gov/pubmed/11802309
- There are no known risks of DHA. It is already provided in infant formulas.
2. From experts in Nutritional Epidemiology – remember the Vitamin D. Consider reading papers about Cod Liver Oil (CLO) and correlation with the risk of diabetes.
- http://www.ncbi.nlm.nih.gov/pubmed/14668274
- http://www.ncbi.nlm.nih.gov/pubmed/27103201
- http://www.ncbi.nlm.nih.gov/pubmed/11043854
3. There was a question about whether or not gluten has a role to play. Every child with T1D and immediate family member should be screened for the autoantibodies for celiac. (http://www.ncbi.nlm.nih.gov/pubmed/23469110)
We gave it a try and his autoantibodies present in August of 2007 were gone in November of 2007.
He stayed on the cocktail and remained negative until 5/2010. About age 14, he thought he knew EVERYTHING and started “pocketing” the supplements. After skipping the DHA, he became autoantibody positive again, and was convinced to go back on the “cocktail”. That worked for a few years, and then he went away to college and chose to skip again. This led to another scare with autoantibody positivity. He went back on the DHA and reversed again, and didn’t have any more autoantibodies for many years. At age 28 he went off the cocktail. He thought the risk was gone and also started on a diet for his heavy weight lifting competitions that was very high in n-6 (lots of bacon). He then tested positive for one of the newly defined autoantibodies (ZnT8). So…the journey continued. He agreed to go back on fish oil (he started taking Dr. Sears’ Zone web site fish oil). This is what I take as well and use the code Poseidon, which is the name of the study for new-onsets. Anyway, good news again – his ZNT8 autoantibody reversed! This roller coaster of watching him go up and down has correlated with HIS choice of whether or not to take supplements. He was told he would become diabetic when he was 11 within “months”, and he just turned 34! So 23 years later, I am glad that he has been willing to give it a try.
My daughter has never been autoantibody positive, even though she has the highest risk profile (identical to her diabetic brother). I bring this up because a child at high risk without autoantibodies may benefit too. With her, we tried DHA plus vaccination for chickenpox and BCG. Who knows what worked – but she has made it to age 28 without autoantibodies.
Resolving inflammation is a problem with a practical solution. We know inflammatory cytokines are elevated prior to the onset of Type 2 diabetes, and inflammation can also predict who develops gestational diabetes. So we looked at how this might affect prevention of T1D. This was just “observational only” , but it is worth a read to see who are the “reverters” and “non-progressors”. (http://www.ncbi.nlm.nih.gov/pubmed/15448085)
The pilot study to look at DHA or placebo in pregnant mothers and infants was called Nutritional Intervention to Prevent (NIP) Diabetes. The DHA was well tolerated, and participants were compliant. Fatty acid and inflammation levels were measured. The study was not powered to show prevention, but it did show that a larger and longer trial could work. (http://www.ncbi.nlm.nih.gov/pubmed/25039804)
This dietary intervention approach meets the “Three Legs of the Stool” which requires that it must be SAFE, EFFICACIOUS and AFFORDABLE in the general population.
Limiting inflammation through the use of high dose DHA has been proven to be safe and effective in other related autoimmune disorders such as rheumatoid arthritis and lupus.
Donate TodayEnroll Now
References
- Hunter P. The inflammation theory of disease. 2012 Nov; 13(11): 968–970.
- Limbert C. Type 1 diabetes – an auto-inflammatory disease: a new concept, new therapeutical strategies. 2012; 10(Suppl 3): I12.
- Bending D, Zaccone P, Cooke A. Inflammation and type one diabetes. 2012 Jun;24(6):339-46.
- Simopoulis AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother 2006;60:502-7.
- Razavi M, Jamilian M, Samimi M, Afshar Ebrahimi F, Taghizadeh M, Bekhradi R, Seyed Hosseini E, Haddad Kashani H, Karamali M, Asemi Z. The effects of vitamin D and omega-3 fatty acids co-supplementation on biomarkers of inflammation, oxidative stress and pregnancy outcomes in patients with gestational diabetes. Nutr Metab 2017;14:80.
- Riachy R, Vandewalle B, Moerman E, Belaich S, Lukowiak B, Gmyr V, Muharram G, Kerr Conte J, Pattou F. 1,25-Dihydroxyvitamin D3 protects human pancreatic islets against cytokine-induced apoptosis via down-regulation of the Fas receptor. 2006 Feb;11(2):151-9.
- Rizzo AM, Montorfano G, Negroni M, Adorni L, Berselli P, Corsetto P, Wahle K, and Berra B. “A rapid method for determining arachidonic:eicosapentaenoic acid ratios in whole blood lipids: correlation with erythrocyte membrane ratios and validation in a large Italian population of various ages and pathologies.” Lipids in Health and Disease 9:7 (2010)
- Chase HP1, Boulware D, Rodriguez H, Donaldson D, Chritton S, Rafkin-Mervis L, Krischer J, Skyler JS, Clare-Salzler M; Type 1 Diabetes TrialNet Nutritional Intervention to Prevent (NIP) Type 1 Diabetes Study Group. Effect of docosahexaenoic acid supplementation on inflammatory cytokine levels in infants at high genetic risk for type 1 diabetes. Pedatr Diabetes 2015;16(4):271-9.
- Ergas D1, Eilat E, Mendlovic S, Sthoeger ZM; n-3 fatty acids and the immune system in autoimmunity. Isr Med Assoc J.2002;4(1)34-38.
- Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-Analysis of the Effects of Eicosapentaenoic Acid (EPA) in Clinical Trials in Depression. J Clin Psychiatry 2011, doi:10.4088/JCP.10m06634.
- van der Wurff ISM, von Schacky C, Berge K, Zeegers MP, Kirschner PA, de Groot RHM. Association between Blood Omega-3 Index and Cognition in Typically Developing Dutch Adolescents. Nutrients 2016, 8, 13; doi:10.3390/nu8010013.
- Stonehouse W, Conlon CA, Podd J, Hill SR, Minihane AM, Haskell C, Kennedy D. DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. Am J Clin Nutr. 2013 May;97(5):1134-43.
- Montgomery P, Burton JR, Sewell RP, Spreckelsen TF, Richardson AJ. Low Blood Long Chain Omega-3 Fatty Acids in UK Children Are Associated with Poor Cognitive Performance and Behavior: A Cross-Sectional Analysis from the DOLAB Study. PLoS One. 2013 Jun 24;8(6):e66697.
- Schaefer EJ, Bongard V, Beiser AS, Lamon-Fava S, Robins SJ, Au R, Tucker KL, Kyle DJ, Wilson PW, Wolf PA. Plasma Phosphatidylcholine Docosahexaenoic Acid Content and Risk of Dementia and Alzheimer Disease. Arch Neurol. 2006 Nov;63(11):1545-50.
- Souied EH, Delcourt C, Querques G, Bassols A, Merle B, Zourdani A, Smith T, Benlian P. Oral Docosahexaenoic Acid in the Prevention of Exudative Age-Related Macular Degeneration. Ophthalmology. 2013 Aug;120(8):1619-31.
- Luxwolda MF, Kuipers RS, Boersma ER, van Goor SA, Dijck-Brouwer DA, Bos AF, Muskiet FA. DHA status is positively related to motor development in breastfed African and Dutch infants. Nutr Neurosci. 2014 Apr;17(3):97-103.
- Bisgaard H, Stokholm J, Chawes BL, Vissing NH, Bjarnadóttir E, Schoos AM, Wolsk HM, Pedersen TM, Vinding RK, Thorsteinsdóttir S, Følsgaard NV, Fink NR, Thorsen J, Pedersen AG, Waage J, Rasmussen MA, Stark KD, Olsen SF, Bønnelykke K. Fish Oil–Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N Engl J Med. 2016 Dec 29;375(26):2530-9.
- Lembke P, Capodice J, Hebert K, Swenson T. Influence of Omega-3 (N3) Index on Performance and Wellbeing in Young Adults after Heavy Eccentric Exercise. J Sports Sci Med. 2014 Jan; 13(1): 151–156.
- Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal Supplementation With Very-Long-Chain n-3 Fatty Acids During Pregnancy and Lactation Augments Children’s IQ at 4 Years of Age. Pediatrics. 2003 Jan;111(1):e39-44.
- Chase HP1, Boulware D, Rodriguez H, Donaldson D, Chritton S, Rafkin-Mervis L, Krischer J, Skyler JS, Clare-Salzler M; Type 1 Diabetes TrialNet Nutritional Intervention to Prevent (NIP) Type 1 Diabetes Study Group. Effect of docosahexaenoic acid supplementation on inflammatory cytokine levels in infants at high genetic risk for type 1 diabetes. Pedatr Diabetes 2015;16(4):271-9.
- Stene LC1, Joner G, Norwegian Childhood Diabetes Study Group; Use of cod liver oil during the first year of life is associated with lower risk of childhood-onset type 1 diabetes: a large, population based, case-controlled study. Am J Clin Nutr 2003;78(6):1128-1134.
- Sørensen IM, Joner G, Jenum PA, Eskild A, Brunborg C, Torjesen PA, Stene LC. Vitamin D-binding protein and 25-hydroxyvitamin D during pregnancy in mothers whose children later developed type 1 diabetes. Diabetes Metab Res Rev 2016;32(8):883-890.
- Stene LC1, Ulriksen J, Magnus P, Joner G. Use of cod liver oil during pregnancy associated with lower risk of type 1 diabetes in the offspring. Diabetologia 2000; 43(9)1093-8.
- Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014 Jan 4; 383(9911): 69–82.
- Superko HR, Superko AR, Lundberg GP, Margolis B, Garrett BC, Nasir K, Agatston AS. Omega-3 Fatty Acid Blood Levels Clinical Significance Update. 2014;8(11):407.
- Rupp H, Wagner D, Rupp T, Schulte LM, Maisch B. Risk stratification by the “EPA+DHA level” and the “EPA/AA ratio” focus on anti-inflammatory and antiarrhythmogenic effects of long-chain omega-3 fatty acids. 2004 Nov;29(7):673-85.
- Gómez Candela C, Bermejo López LM and Loria Kohen V. Importance of a balanced omega 6/omega 3 ratio for the maintenance of health. 2011;26(2):323-329.
- Simopoulos AP. Omega–6/Omega–3 Essential Fatty Acids: Biological Effects. 2009, vol 99, pp 1–16.


 Vitamin D
Vitamin D